
Duke University School of Medicine, in collaboration with the UNC School of Medicine, has been awarded a U24 grant (U24NS135250) by the National Institute of Neurological Disorders and Stroke (NINDS) for StrokeNet, entitled “Duke-University of North Carolina (UNC) Eastern North Carolina and Southern Virginia RegIonal Stroke trIal cONsortium (ENVISION)”.
Duke Neurology’s Wayne Feng, MD, division chief of stroke and vascular neurology, serves as the contact principal investigator. Duke Emergency Medicine’s Alexander Limkakeng, MD, and UNC’s David Hwang, MD, division chief of neurocritical care, act as co-principal investigators. Duke Neurology’s William Powers, MD, will be the training director.
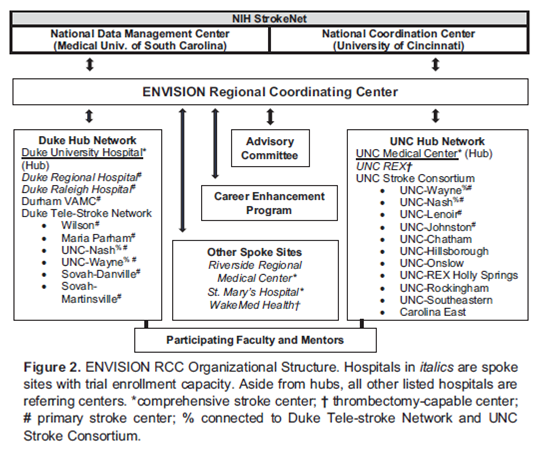
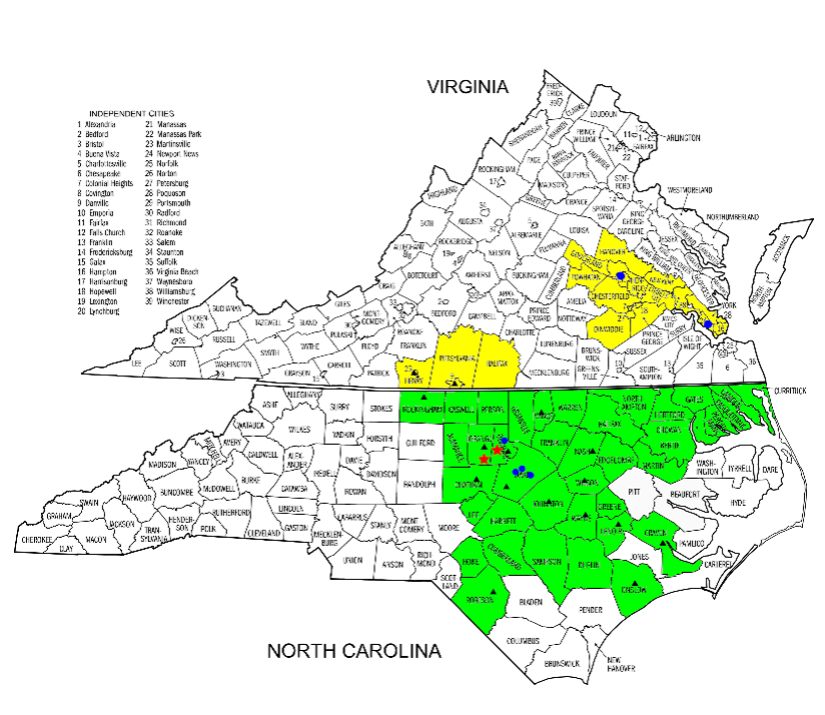
This award will provide over $2.7 million in total cost over the next five years to establish and oversee a regional network of hospital sites in eastern NC and southern Virginia. Most of these sites are in rural areas to provide stroke trial options in acute treatment, prevention, and recovery to stroke patients where standard care is not available.
This local consortium will draw stroke patients from a region of 2.2 million people. The area includes six major hospitals and 21 regional hospitals with total stroke admissions of more than 8,000 people per year. The consortium also supports one StrokeNet fellow who is interested in conducting stroke clinical trials.
Dr. Feng and Dr. Limkakeng are excited about this federal grant and the health impact it promises to the region.
“This ENVISION grant provides a great opportunity for several institutes to work together to establish a regional stroke trials consortium in eastern North Carolina and southern Virginia — part of “the stroke belt” with disproportionally higher stroke incidence and stroke mortality than other U.S. regions,” Dr. Feng said. “Stroke patients, especially from rural areas, now have hope to access to various stroke clinical trials in acute treatment, prevention and recovery within the network.”
Dr. Limkakeng adds, “As an emergency physician, I have seen the devastating impact that a stroke can have on patients and their families. I am honored to help lead this interdisciplinary effort to improve outcomes from stroke and provide advanced therapies to previously underserved populations.”
About StrokeNet
NINDS established the NIH StrokeNet to facilitate the development, promotion, and conduct of high-quality, multi-site clinical trials focused on key interventions in stroke acute treatment, prevention, and recovery.
An additional goal of the StrokeNet is to educate future stroke investigators and researchers in stroke. The educational core provides webinars focused on stroke, research methodology, and professional development.
This network currently has a network of 27 regional coordinating centers, involves approximately 500 hospitals across the United States, and is designed to serve as the infrastructure and pipeline for new potential treatments for patients with stroke and those at risk of stroke. Currently, 11 ongoing landmark trials are actively enrolling stroke trial participants.
